Tumor heterogeneity & spatial organization of the anti-tumor immune response
Tumor Heterogeneity: The problem
As tumors grow, they also evolve and become heterogeneous. Tumor evolution has been likened to Darwin's evolution of species. While tumors, in most cases, originate from a single mutant cell, as they grow, daughter cells will acquire additional mutations. As these daughter cells expand with their new mutations, the result is a tumor which is a patchwork of different populations—a genetically heterogeneous tumor, with different regions of tumor carrying distinct mutations.
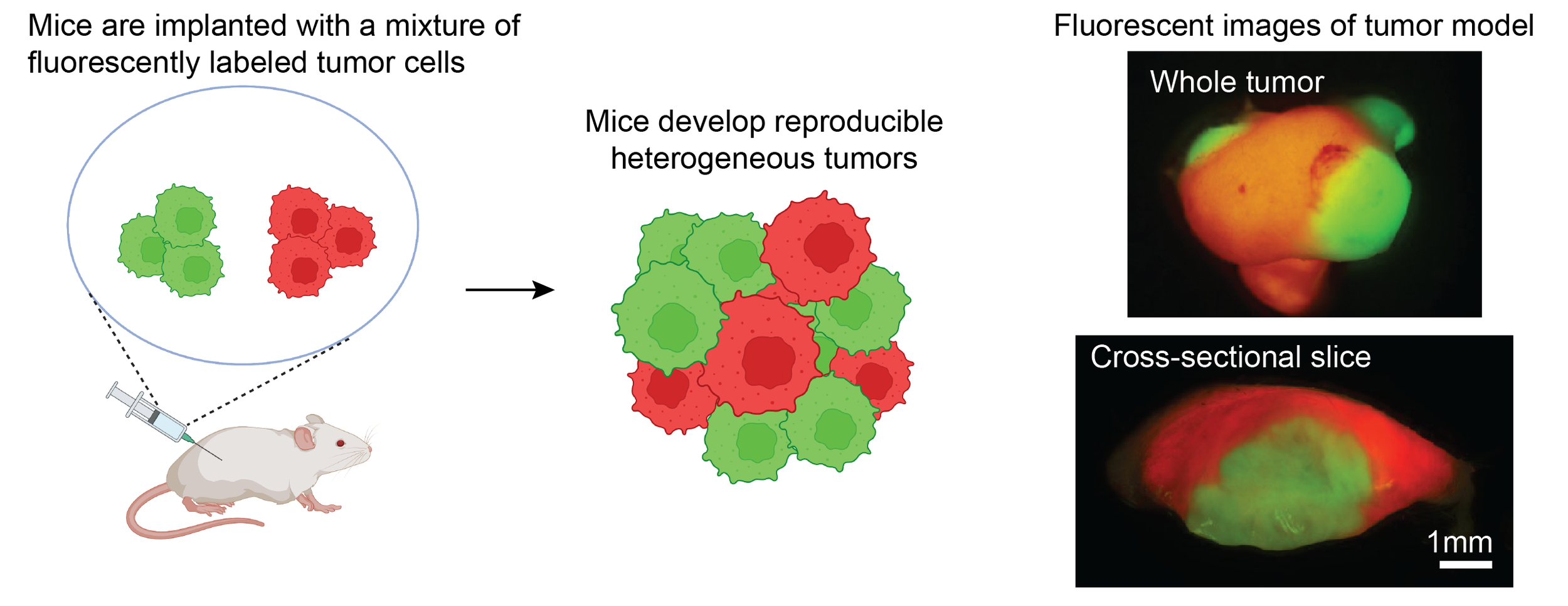
Patients with heterogeneous tumors have worse outcomes, including poorer responses to immunotherapy. To study the mechanisms linking heterogeneity to inferior outcomes, we have established a novel platform to model tumor heterogeneity. We make use of 11 cell lines we established from carcinogen-induced skin squamous cell carcinomas, which we have named the CIT (carcinogen-induce tumor) cell lines. These cell lines carry a high but physiologically relevant mutation burden, and exhibit a range of reproducible immune infiltrates when implanted in mice. We have introduced fluorescent tags into these cell lines, enabling us to mix them and implant mice with reproducible heterogeneous tumors in which constituent populations can be precisely tracked and individually modulated.
Impact of TUMOR HETEROGENEITY on the Immune Response
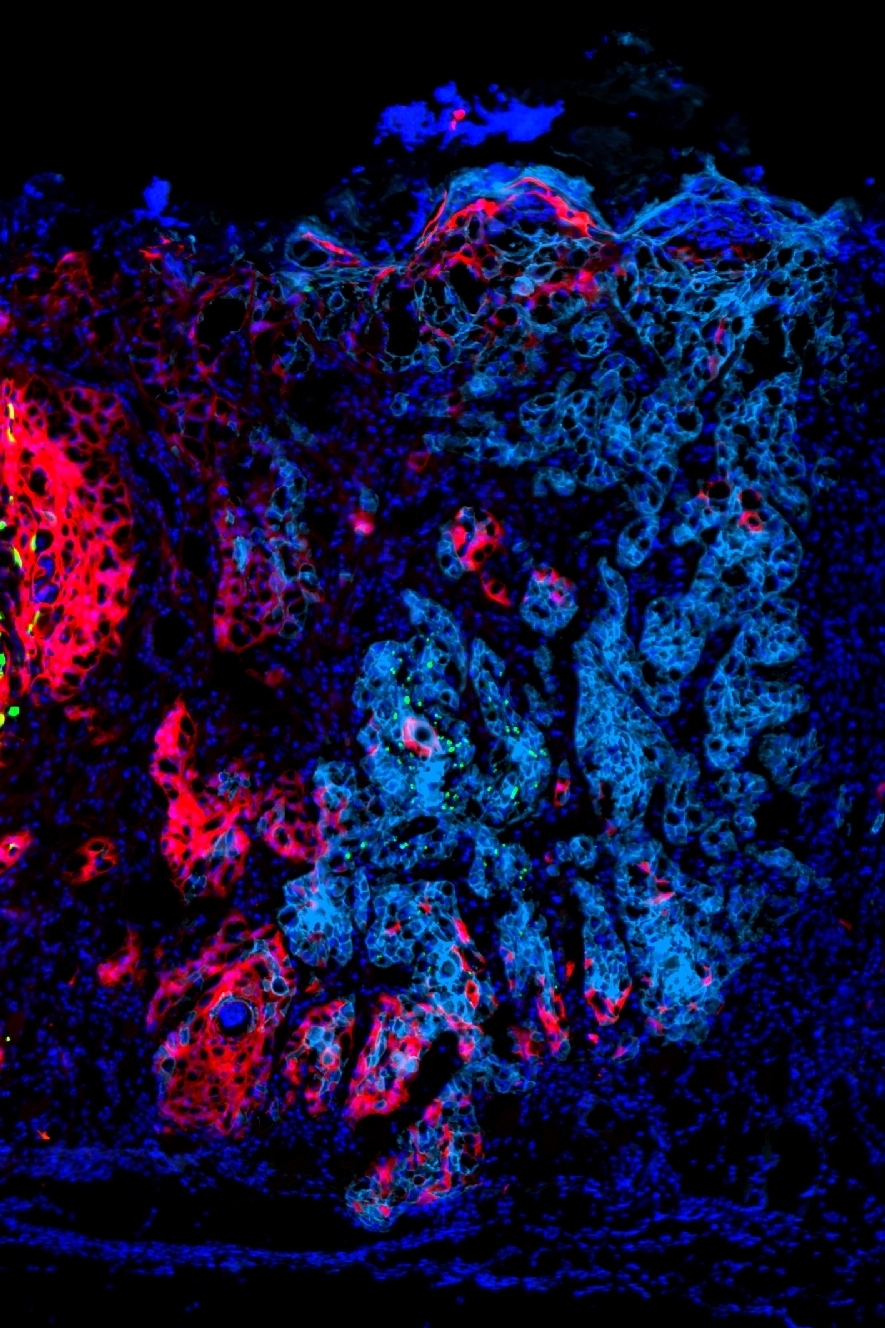
We have discovered that tumor cells drive the abundance and functional activity of immune cells on a highly localized spatial scale. We have established models of heterogeneous tumors comprised of either (1) an “immune hot” plus an “immune cold” tumor populations, or (2) two “immune hot” populations, and labeled the tumor populations with YFP (green) and RFP (red) fluorescent markers. In both cases, we find that the immune microenvironment adjacent to red versus green tumor cells is distinct, and T cell activity is unequal between tumor regions. Mechanistically, we find that “cold” tumor cells produce CX3CL1 to locally recruit macrophages and dampen T cell activity in their vicinity. We are currently investigating the effect of tumor-produced CX3CL1 on the tumor microenvironment, as well as identifying additional factors that tumor cells use to drive the spatial organization of immune cells within the tumor.
An effective anti-tumor immune response is dependent on the ability of T cells to recognize and attack tumor cells. T cells can identify tumor cells by recognizing tumor neoantigens, which are the result of mutations in the tumor cell. However, tumor neoantigens display the same spatial heterogeneity that mutations do. We are currently extending our model system to study how T cells respond to spatially restricted neoantigens.
Additional questions we are interested in include:
How does a heterogeneous tumor evolve after treatment with immunotherapy? Can immunotherapy productively alter “bad” patterns spatial organization of T cells in heterogeneous tumors?
How does tumor composition, i.e., ratio of “hot” to “cold” tumor cells, influence the overall anti-tumor immune response and the spatial organization of immune cells? Having shown that tumor cells shape the organization of immune cells, does immune pressure also conversely influence the spatial organization of tumor subpopulations?
What rules govern how the immune system will respond to a heterogeneous (e.g., subclonal) tumor antigen? How well do T cells find their cognate antigen in a spatially complex field of antigens?